A popular prescription anti-anxiety drug is being recalled nationwide for a “potentially life-threatening” error in the packaging.
Pennsylvania-based drugmaker Endo announced this week that it is expanding the recall of clonazepam tablets because some boxes have the wrong drug strength and the wrong drug code.
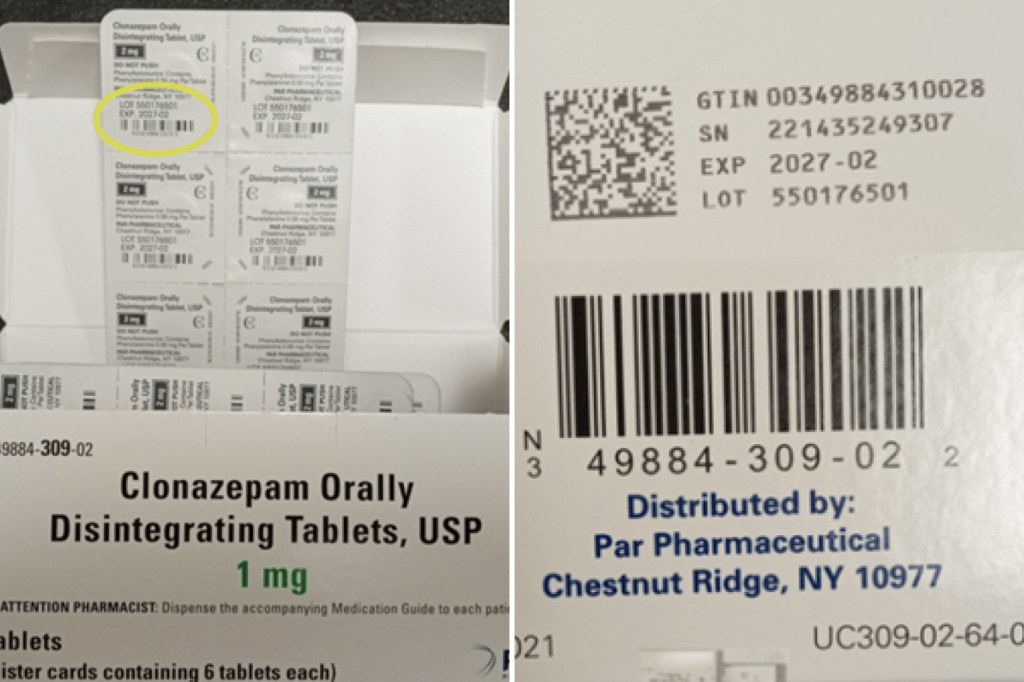
The new recall affects 16 lots of Clonazepam Oral Disintegrating Tablets, USP (C-IV), with dosages ranging from 0.125 milligrams to 2 milligrams. Packs contain 10 blister strips each containing six tablets. They will expire between August 2026 and February 2027.
Clonazepam is a benzodiazepine used to treat panic disorders and some types of seizures.
Endo warns that consuming a higher dose of clonazepam may increase the risk of drowsiness, confusion, dizziness, decreased reflexes, and loss of muscle control or strength.
There is also a risk of “significant, possibly life-threatening” breathing problems, especially for those with respiratory disease, those prescribed the maximum dose and those taking other medications that affect breathing.
Endo reports that as of Monday, it has received no reports of problems stemming from the recall. The pharmaceutical company announced its initial recall in July of only one quantity of clonazepam.
At the time, Endo blamed an “error on a third-party packager” for the mislabeling.
Some cartons listed the strength of the product as 0.125 mg rather than 0.25 mg. The bubble strips inside the package reflected the right strength, Endo said.
The recalled boxes also list Par Pharmaceutical of New York as a distributor. The company, based in Chestnut Ridge, marketed clonazepam before the product was acquired by Endo.
People with questions about the recall should call (855) 589-1869 or email rxrecalls@inmar.com.
Retailers with these products are being instructed not to sell them, and consumers not to take them. Consult a doctor if you believe you have received an incorrect dose of clonazepam.
#Clonazepam #recalled #potentially #lifethreatening #error
Image Source : nypost.com